
pH-Correct Creatine® Monohydrate

KRE-ALKALYN DOMINATES
Kre-Alkalyn EFX...it's Buffered. It's Stable.
Kre-Alkalyn® EFX represents a major breakthrough in performance supplementation thanks to its multi-patented, pH-Correct creatine® stabilization technology. Our Scientist Dr. Jeff Golini discovered that “buffering” to pH-12 produced a stable creatine monohydrate molecule.*
pH-Correct Makes it a Category of One!
Kre-Alkalyn® EFX is the only creatine monohydrate product with a pH of 12 because its molecules are synthesized with ‘buffering’ agents using a patented (#6,399,661) manufacturing process to make it pH-Correct.*
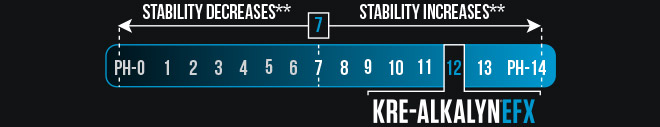
Creatine stability is negatively influenced by acids and liquids at pH 6.9 or less. (#6,399,661). Kre-Alkalyn® is the only creatine with patent protection from pH 7 to pH 14.*


NO ONE TESTS LIKE

Ingredients either Pass or Fail...and a 'Fail' is rejected and shipped back.
When you invest in EFX Sports products, you are putting your faith in the efficacy and purity of our ingredients, and the integrity of our brand. That's why we NEVER take that trust for granted.
From dock-to-dock, we own every phase and process to ensure that what's on the label is what's in the bottle...without question.
PHASE 1
INCOMING RAW MATERIAL TESTING
-
IDENTITY
To confirm the ID of a given Raw Material and the accuracy of the Certificate of Analysis (COA) we use FTNIR (near-infrared technology). -
PURITY
Vendor COA claims are checked for purity by HPLC, GC, or ICP. -
Heavy Metals
We test for Arsenic, Cadmium, Lead, and Mercury. Heavy Metals must be under the USP specified guidelines. -
MICROBIAL
We test for Total plate count, Total Yeast and Mold count, and Coliform bacteria to make sure that the Raw Material complies with the USP, AHPA, and FDA guidelines. -
PATHOGENS
Raw Materials are tested for E. coli, Salmonella, and Staphylococcus aureus pathogens (as needed). -
Drug Screen
Raw Materials are tested for banned steroidal substances (as needed).
PHASE 2
DURING PRODUCTION TESTING
-
CLEANLINESS
We perform ATP swab tests prior to batch production and allergen swab tests as needed to ensure equipment is free of potential contamination or microbes. -
IDENTITY CHECK
We use FTNIR to confirm the ID of a given in-process mix to ensure it contains the correct raw ingredients. -
PURITY CHECK
Purity is checked by using HPLC, GC, and/or ICP to ensure the in-process mix will create a final product that meets label claims.
PHASE 3
FINISHED PRODUCT TESTING
-
IDENTITY CHECK
We use FTNIR to confirm the ID of a Finished Product. -
Purity (label claim)
We check the purity by HPLC, GC, and/or ICP if there is a label claim on the Finished Product COA. -
MICROBIAL CHECK
We test for Total plate count, Total Yeast and Mold count, and Coliform bacteria to make sure that the Finished Product complies with specifications. -
PATHOGENS CHECK
We test again for the following pathogens, E. coli, Salmonella, and Staphylococcus aureus (as needed). -
ALLERGENS
We list the allergens if present in the Finished Product. -
HEAVY METALS CHECK
We test the Finished Product for Heavy Metals contaminants (Arsenic, Cadmium, Lead, and Mercury) and make sure they are under the USP specified guidelines.
DON'T SETTLE FOR LESS
This shop is powered by Shopify
